Transforming Compliance through Digital Innovation

About DSS
At Validation Resources Group (VRG), our Digital Solution Services division helps medical device manufacturers—and beyond—transition to smarter, validated, paperless systems. We merge regulatory expertise with modern platforms to deliver scalable, GxP-compliant applications.

Our Core Services
Digital Integration Initial Assessment
Application Development
CSV Validation & Testing
Quality & Compliance Support
Technology Partners
Build interactive, intuitive applications that drive productivity—no coding needed. Use pre-built templates or start from scratch. Manage and govern app development and use on the production floor.
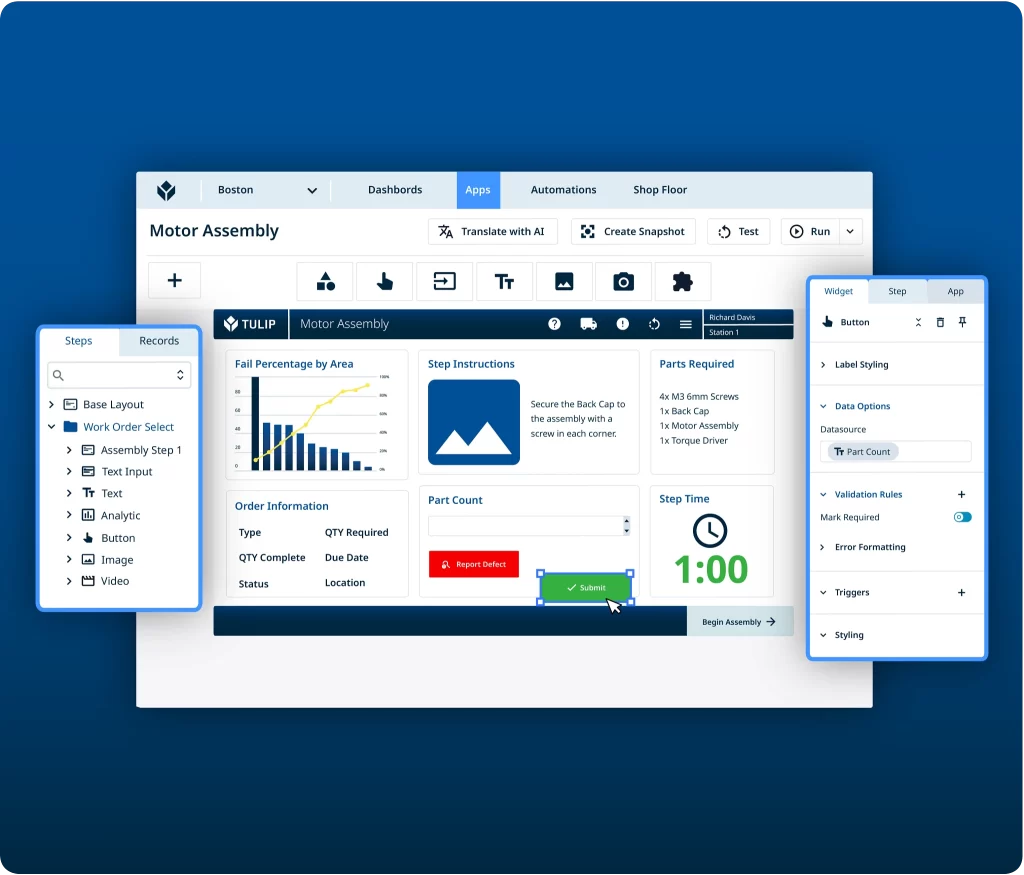
Electronic Device History Records (eBR / eDHR)
eLogbooks
CAPA & NC Tools
Dashboards
History Reports
Material Used Viewer
Modular Applications
Industries We Serve
Medical Device
Biotech & Pharma
Regulated Manufacturing
Why Work With VRG Digital Solution Services
Compliance + Validation Expertise
Strategic SaaS Partnerships
Certified QA & CSV Specialists
About DSS
At Validation Resources Group (VRG), our Digital Solution Services (DSS) division supports medical device manufacturers and other regulated industries in their transition to smarter, validated, and paperless systems.
We combine deep regulatory knowledge with innovative digital platforms to deliver scalable, compliant, and highly functional applications across the manufacturing and quality lifecycle.
Our Core Services
- Digital Integration Initial Assessment
- Evaluate your digital maturity level
- Identify automation opportunities
- Build your roadmap for smart manufacturing
Application Development
- Build no-code / low-code apps on Tulip, etc
- ERP & equipment connectivity
- UI/UX design tailored to operators
App CSV Validation & Testing
- Full Computer System Validation lifecycle (GxP)
- Documentation with traceability matrices (Tulip)
- Testing protocols (IQ, OQ, PQ)
Quality & Compliance Support
- Digital CAPA and NC workflows
- eLogbook implementation
- QA dashboards and KPI reporting
Technology Partners
We work with trusted platforms to deliver results: Tulip Interfaces – No-code apps, eDHR, eLogbooks
Use Cases We Solve
eDHR (Electronic Device History Record)
- Paperless manufacturing records
- Real-time data capture and approvals
- ERP and equipment integration
eLogbooks
- Digital logs with timestamp and audit trail
- Replace paper-based GMP logbooks
- Configurable workflows
CAPA & Non-Conformance Tools
- Structured QA workflows
- Dashboards for monitoring and reporting
- Integrated with QMS or as standalone
Industries We Serve
- Medical Devices
- Pharmaceutical & Biotech
- Food & Beverage
- Regulated Manufacturing
Why Work With VRG Digital?
- 20+ years of industry compliance expertise
- Strategic partnerships with best-in-class platforms
- Certified professionals in CSV, QA, UI/UX, and validation
- Deep experience in GxP, FDA, and ISO standards
